Drugs Under Development
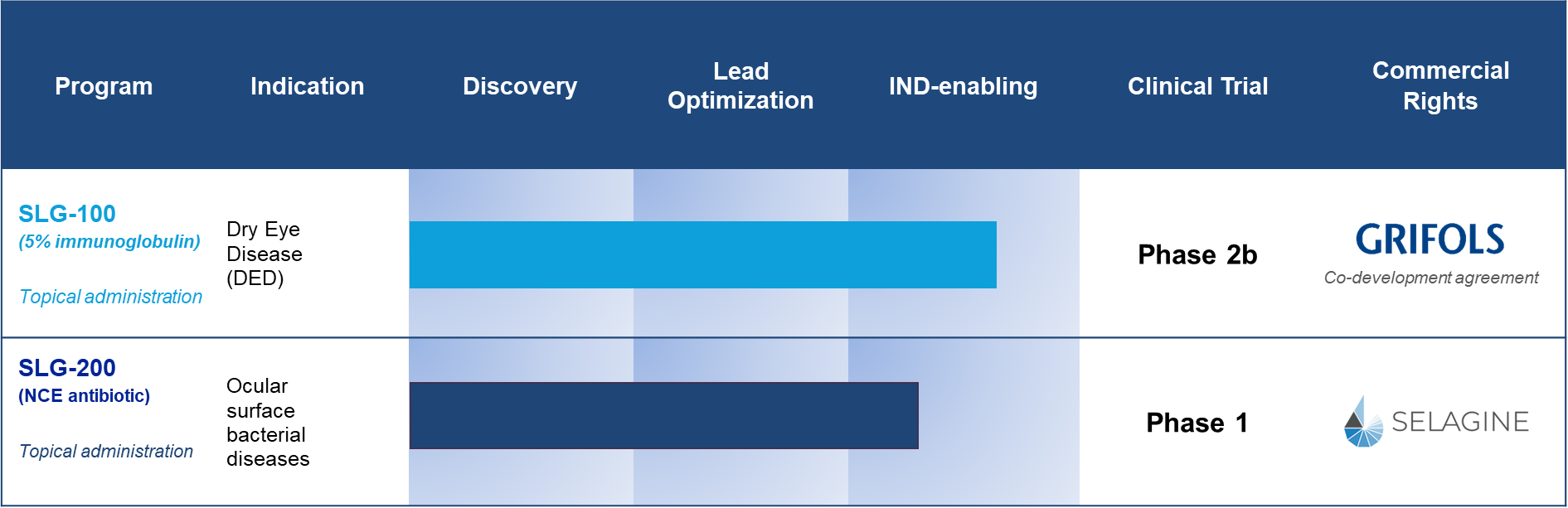
SLG-100 - A Clinical Stage Therapeutic Product For Treating Dry Eye Disease (DED)
In the USA 17 million patients have been diagnosed with Dry Eye Disease (DED). As many as 5 - 35% of patients visiting an ophthalmology clinic report DED symptoms, making DED one of the most common conditions seen by ophthalmology specialists. The global DED market was estimated at 4.5 billion USD in 2020, covering prescription drugs, OTC drugs and medical devices. DED is the 3rd largest indication of the overall ophthalmology market. The largest segment of the DED market are artificial tears, which comprise more than 50% market share with estimated 2.6 billion USD. Therefore, there exists an unmet need for effective prescription therapeutics for DED.
Current therapeutic strategies for DED focus on breaking the T-cell perpetuated inflammatory cycle and has led to the development of drugs that target them. There are three FDA approved drugs currently available for treating patients with DED - Cyclosporine 0.05% (Restasis), Cyclosporine 0.09% (Cequa) and lifitegrast 5% (Xiidra). All three FDA approved DED drugs have in common that they affect T-cell function and do not have any direct actions against DED-specific inflammatory proteins (cytokines) or pathogenic autoantibodies.
SLG-100 eye drops shift the current paradigm of DED treatment that focuses on T-cell mediated inflammation as central to the pathophysiology to also include direct neutralizing actions against DED-specific inflammatory proteins (cytokines) and autoimmune inflammation that is driven by post-translational modifications in self-proteins (citrullination) and autoantibodies (ACPAs). In contrast to the three FDA approved DED drugs that affect T-cell function, SLG-100 is a biologic comprising pooled human immune globulins that have natural anti-cytokine antibodies and anti-idiotypic (anti-immunoglobulin) antibodies with specific actions against DED-specific cytokines and autoantibodies, and other anti-inflammatory actions on neutrophils, dendritic cells, T-regulatory cells and complement system. Thus SLG-100 has broad-spectrum anti-inflammatory actions via molecular and cellular mechanisms.
SLG-100 is a clinical stage product. We have performed a pilot clinical trial to determine the preliminary therapeutic potential and safety of SLG-100 eye drops in patients with DED. As compared to Vehicle treatment, SLG-100 eye drops twice a day for 8 weeks caused significant reduction in signs and symptoms of DED with no difference in tolerability or adverse events.
SLG-200 - A Novel Ophthalmic Antibiotic for Treating Bacterial Infections
Globally, corneal opacity, most caused by infectious keratitis, is the fifth most prevalent cause of blindness, with the primary risk factors being contact lens wear, ocular injury, and ocular surface disease. In the USA, infectious keratitis accounts for approximately 1 million visits to health professionals and 58,000 emergency department visits annually. This results in a substantial economic impact, with direct health expenditures of 175 million USD. Among the various causal organisms, Staphylococcus aureus, Streptococcus pneumoniae, and Pseudomonas aeruginosa are the most frequently identified bacterial species. Many of these bacteria also have a high prevalence of resistance to currently available antibiotic eye drops. For example, methicillin-resistant S. Aureus (MRSA) is one of the most common causes of bacterial keratitis; however, 93% of MRSA are resistant to azithromycin, 84% are resistant to erythromycin, 76% are resistant to ciprofloxacin, and 66% are resistant to moxifloxacin. In contrast, only 24% MRSA are resistant to SLG-200. No new antibiotic eye drop has been approved in the USA over the past 15 years. Thus, an unmet need exists for new, potent antibiotics.
SLG-200 is an ophthalmic-only antibiotic eye drop. It is a new chemical entity (NCE) that has been designed specifically to overcome resistant priority pathogens. It is highly potent against key susceptible and resistant Gram-positive and intracellular pathogens including Staphylococcus aureus (MRSA), Streptococcus pneumoniae (PRSP), Coag-Neg Staph (CoNS), Neisseria gonorrhoeae, and Chlamydia trachomatis. SLG-200 is being exclusively formulated for topical ophthalmic use. Since its administration is solely topical, resistance to SLG-200 has the potential to be lower than antibiotics that are administered both topically and systemically.


